Thank you! Your submission has been received!
Oops! Something went wrong while submitting the form.
Discover Axon.R – a GxP-aligned, audit-ready R package validation and maintenance framework so your teams can safely use open-source R in clinical submissions. Replace months-long package onboarding with a repeatable process that can run in hours.
Open-source tools like R give clinical teams flexibility and access to advanced methods. But in regulated environments, package validation often becomes slow, manual, and tightly coupled to environment releases consuming time, attention, and coordination across teams.
3+ months to onboard a single package – coordinating biostats, programmers, QA, IT, and documentation
Delays in analyses, study milestones, and ultimately regulatory submissions
No central view of which packages are approved, under what conditions, and for which use cases
Teams forced to fall back to legacy tools even when better open-source options exist
Instead of treating each R package as a one-off validation project, Appsilon helps you implement Axon.R, a standardized validation and maintenance tool and framework based on R Validation Hub best practices and extended for regulated use.
It combines risk-based assessment, controlled validation runs, containers as a validation method and audit-ready evidence.
A single, controlled process for assessing and validating public and internal R packages. Axon.R supports metric-driven scoring paired with manual risk classification informed by context-of-use.
Validation is run in a validated execution environment, producing consistent outputs and traceable evidence for audits and internal QA.
Risk-level dependent approval flows and structured remediation when checks fail or evidence is incomplete so nothing is “approved by accident." Includes multi-step approvals for high-risk packages.
When a package gets approved, Axon.R can publish it to a validated repository (e.g., Posit Package Manager) and retain the key metadata and audit trail
Supports integrations with CI/CD, SSO, and other enterprise systems.
Plug into any end user environment (Posit PM, Container Images, and more).
You retain full ownership of the framework, code, and documentation. We train your biostats, programming, and platform teams to run and build on top, with optional support from Appsilon.
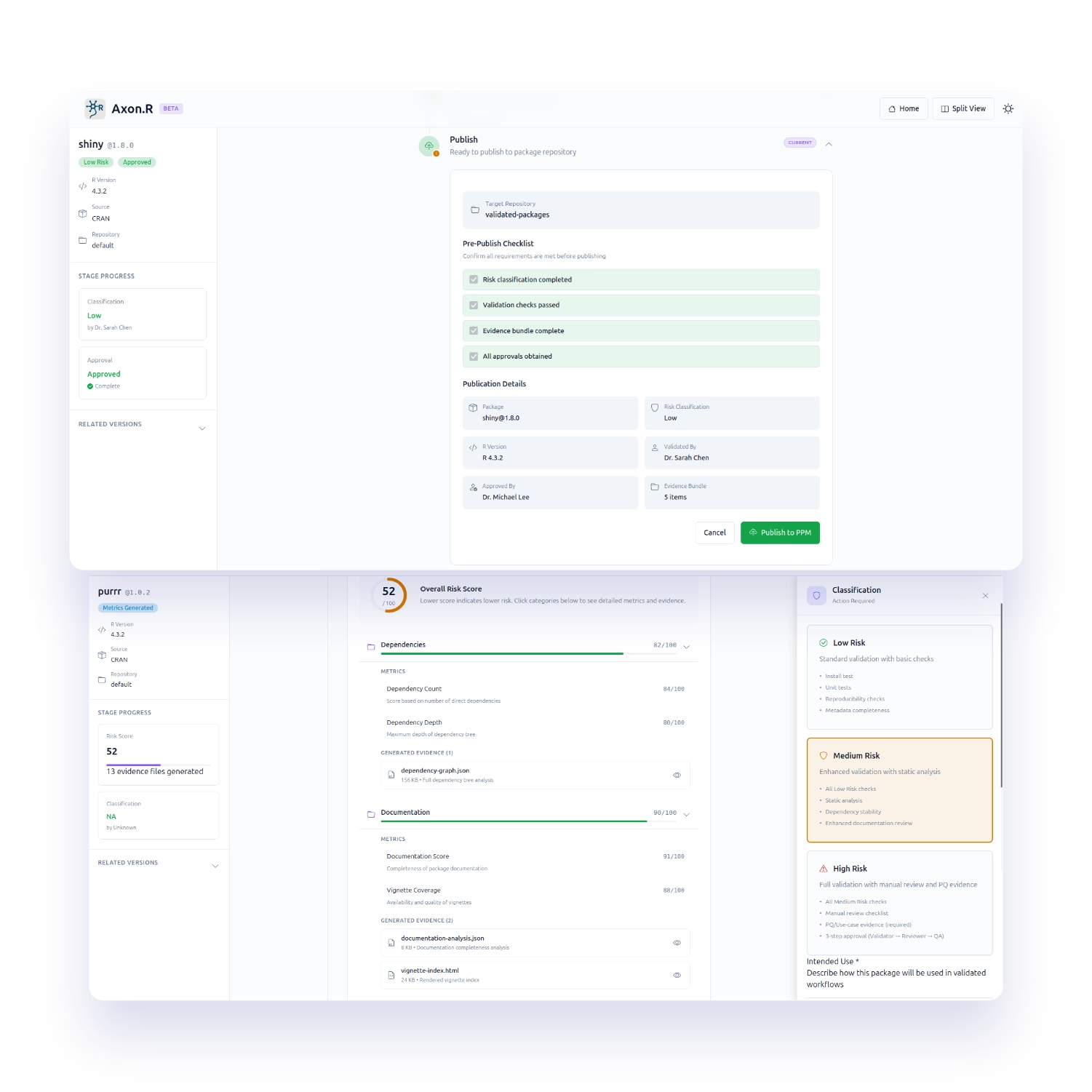
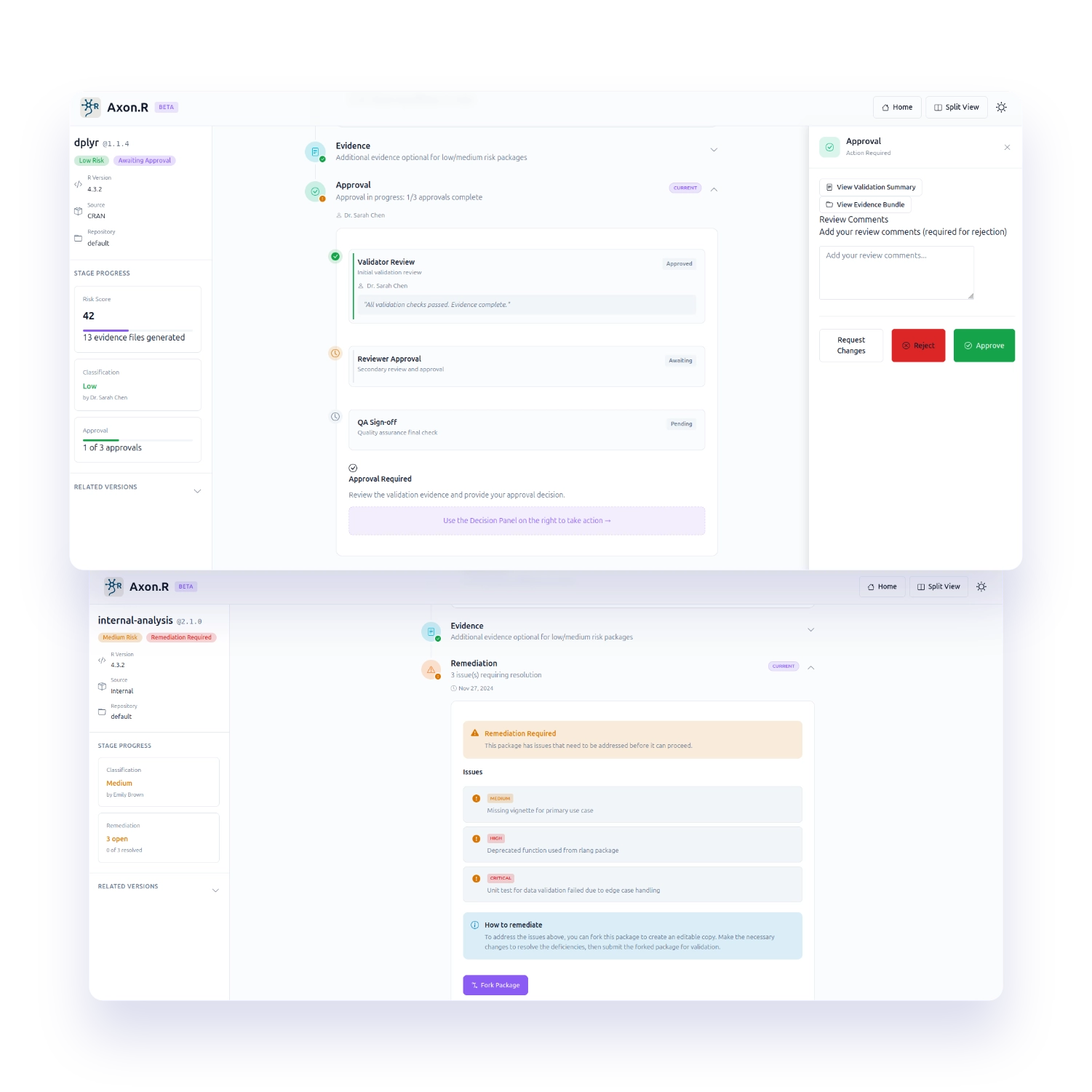
Axon.R takes packages from public or internal sources and automatically generates metrics to classify risk in the context of use. It then runs validation in a validated execution environment, produces an evidence bundle for approval, and publishes validated packages to your approved repository with full version lineage tracking.

Your timeline depends on the current environment (SCE/repository setup, access, integrations) and internal approval cycles (QA/Compliance). That’s why we run delivery as a milestone-based rollout with clear checkpoints.
Mapping your current environment, risks, and package portfolio. Risk-based validation approach, draft documents, and target scope.
Configuring and setting up Axon.R in your environment. Automating key checks and validating an initial set of priority packages.
Complete validation documentation, training your teams. Working framework in place, hand over of code and SOPs, support for audit readiness.
Ongoing help with upgrades, new packages, and framework enhancements as your needs grow.















Data Engineering Lead
Top 10 Pharma Company
Associate Director
Top 50 Pharma Company
Human Resources People Partner
Top 10 Pharma Company
A trusted partner to leading pharmaceutical and life sciences organizations worldwide.
Out of 15,000+ applicants each year, only 1% are selected to join our team - a diverse group of programmers, managers, biostatisticians, and PhD-level scientists actively contributing to the industry.
With over 150 projects delivered for pharmaceutical companies, you gain access to our deep, collective expertise and training support from experienced delivery managers, biostatisticians, programmers, and scientists.
Our team members are creators and contributors to open-source frameworks such as Rhino, aNCA, teal, and many more—collectively downloaded nearly 500,000 times. We are active participants in Pharmaverse and R Consortium working groups.
8 out of the 10 world’s largest pharmaceutical companies have trusted our programmers and statisticians to support AI-driven workflows, analyse all types from data: from clinical and real-world data (RWD) to drug discovery experiments.
Find clear answers to common questions about GxP compliance, helping you navigate regulations with confidence.
R package validation is the process of demonstrating that the packages used in regulated workflows behave reliably and that results are reproducible and appropriately controlled. It matters because regulators require traceability, accuracy, and confidence in the statistical results produced.
FDA’s Statistical Software Clarifying Statement confirms that any software, including R, is acceptable for submissions as long as it is documented, reproducible, and appropriately controlled.
Our teams can implement Axon.R in 8–12 weeks, depending on existing quality processes, governance maturity, automation readiness and integration needs.
Axon.R is designed to turn validation into a repeatable workflow that can be completed in hours or days (depending on package risk level, evidence requirements and human processes), rather than being blocked by multi-month release cycles. Typically within the industry, we see system releases and outdated processes being a particular bottleneck for packages to be released. With Axon.R you can have packages released in under a week.
Most teams are slowed down by manual workflows (documentation, reviews, approvals, and re-validation triggered by environment changes) coupled with outdated or fragmented processes and tooling that slow down documentation, dependency checks, and reproducibility verification.
Package validation is only one layer; full compliance usually requires governed infrastructure, controlled environments, auditability, and lifecycle management. This is typically accomplished through a broader Statistical Compute Environment (SCE).
From custom dashboards and applications to AI-powered solutions and compliant computing environments, our engineers and infrastructure architects accelerate clinical development within fully validated, regulatory-compliant frameworks.