Thank you! Your submission has been received!
Oops! Something went wrong while submitting the form.
The premier Statistical Computing Environment designed for next-gen pharma stack. Unify R, Python, and SAS in one secure, validated platform without the legacy infrastructure burden.
Standardize how R, Python, and SAS workflows run across teams with full traceability and automated audit readiness.
Remove delays caused by legacy tools and enable automation, reproducibility, and AI-ready workflows from day one.
Clinical teams must deliver faster, compliant insights - yet many still work within fragmented, outdated systems that slow study timelines and increase operational risk. A modern SCE unifies everything into one secure and validated platform.
Request Consultation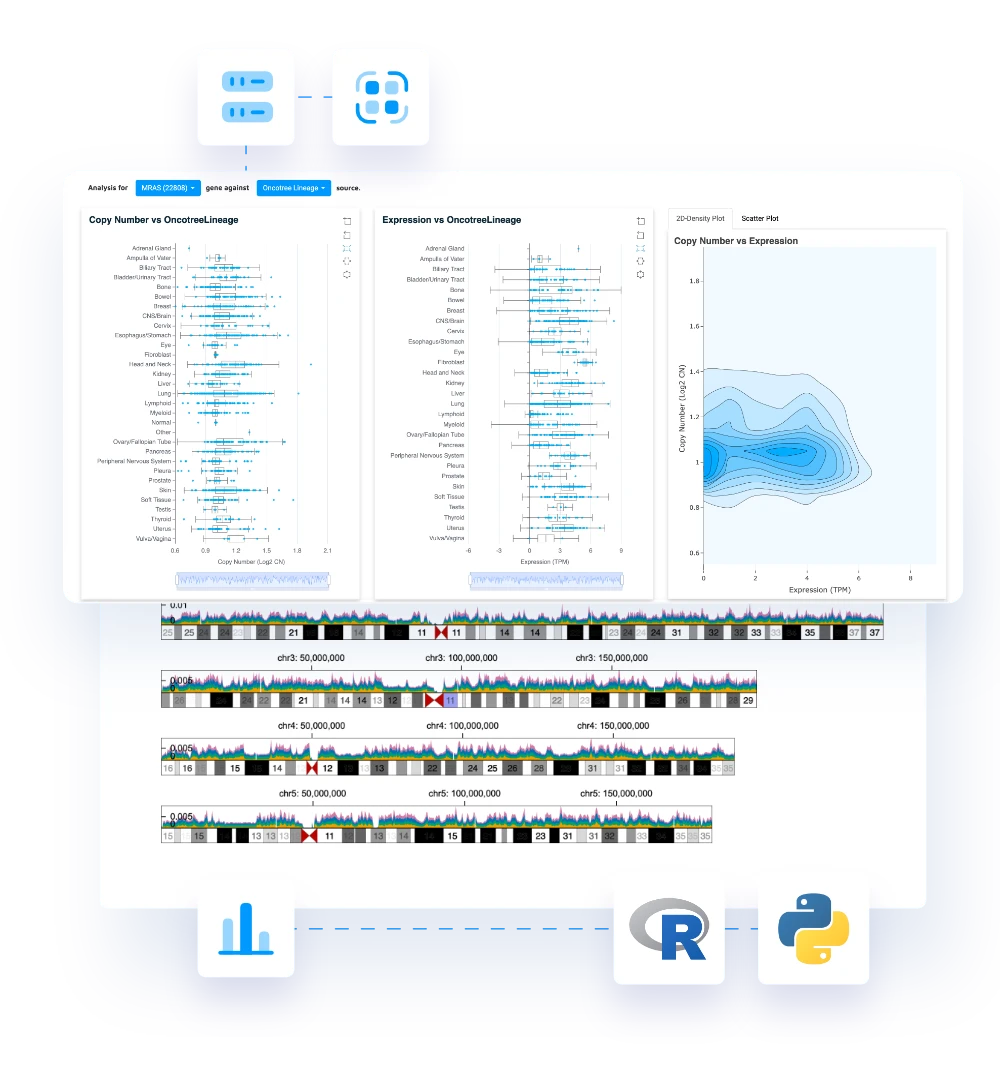
A modern SCE replaces fragmented workflows with one validated, scalable environment for R, Python, and SAS. It standardizes analytics, strengthens compliance, and accelerates delivery across the entire study lifecycle.
A validated SCE centralizes logs, versioning, and traceability so teams stay inspection-ready at all times.
DEPLOY
A validated, reproducible clinical environment deployed on your infrastructure with full GxP documentation.
INTEGRATE
Your SCE regulatory systems, enabling automated and standardized clinical data workflows.
CONTACT US
Your environment can be tailored to specific workflows, governance requirements, and enterprise scale.
EXTEND
Extend your SCE with validation tools, reproducible R/Python containers, QC modules, and analytics apps - using Appsilon add-ons or your own.
TRANSFORM
Gain structured SAS-to-R/Python migration support with focused training and adoption guidance for your teams.
SUPPORT
Keep your SCE compliant and up to date with ongoing maintenance, validation refreshes, and environment enhancements.
Read how Appsilon helped a top 50 pharmaceutical company design a custom system for analytics in R and Python, saving the client $930,000 annually.










Generate TLFs faster, validated R submissions.
Use Modern Tools. Explore with multiple languages, submit in R.
Streamline clinical trial tasks from preprocessing to reporting for faster, error-free results.
Secure, scalable, reproducible infra; no hidden “shadow systems.”; Fast change management with IaC
Learn how pharma companies are building validated, flexible platforms to support both regulatory submissions and modern analytics.





Data Engineering Lead
Top 10 Pharma Company
Associate Director
Top 50 Pharma Company
Human Resources People Partner
Top 10 Pharma Company
From custom dashboards and applications to AI-powered solutions and compliant computing environments, our engineers and infrastructure architects accelerate clinical development within fully validated, regulatory-compliant frameworks.