How Global Pharma Leaders Use Open Source and Why It Levels the Playing Field for Everyone
Big pharma is moving to open source. The era of million-dollar licensing is slowly coming to an end. Mid pharma should follow that trend, or they’ll risk falling behind.
Companies like Roche, Novo Nordisk, and GSK are using R for actual FDA submissions. Even Python is getting mentioned more and more. These global leaders have proved open source works for regulatory workflows, and they're moving fast. Mid-size pharma companies face the same compliance requirements but can't justify the same software budgets.
But here’s what most decision-makers don't realize: open source isn't reserved for companies with deep pockets and teams of data scientists. It lets smaller teams deliver validated submissions without the enterprise price tag.
This article breaks down how global pharma leaders use open source, why it levels the playing field for everyone, and what you need to make it work at your company.
What Global Leaders Show Us About Open Source
Industry leaders have already proved that R is fast and transparent enough for regulatory submissions and approvals.
Companies like GSK, Novo Nordisk, and Roche are running validated open source pipelines for actual FDA submissions. They've done the hard work of proving it works, and now mid-size pharma can follow the blueprint.
GSK
GSK set an ambitious target: 75-100% open source by 2025.
They invested in retraining teams, built validation frameworks, and established an R Center of Excellence. All of that resulted in faster analysis, improved recruitment, and reduced vendor dependency. GSK restructured their entire analytics operation around open source.
Novo Nordisk
Novo Nordisk made history with the first R-based FDA submission.
They ran R and SAS in parallel during validation to prove equivalence. This approach showed regulators that open source meets the same standards as proprietary software. The FDA accepted it without issue.
Roche
Roche built an end-to-end R submission pipeline using packages like {admiral}, {teal}, and {rtables}.
Their system handles regulatory submissions and exploratory work. Everything is validated, auditable, and reproducible. Roche proved you don't need separate systems for compliance and innovation - R handles both.
What does this mean for small and mid-sized pharma?
The bottom line is this: open source is proven at the highest level.
Smaller pharma companies don't need to guess where the industry is headed. Global leaders have already validated the technology, built the frameworks, and shown regulators it works. You're not taking a risk by adopting open source. You're just following established best practices.
The Great Equalizer - Open Source for Every Pharma Company
Open source removes the entry barriers that kept smaller pharma companies stuck with expensive legacy systems.
Mid and small-size pharma can use the same tools, packages, and validated workflows as big players - without the enterprise price tag.
Why open source levels the playing field
Here's what changes when you adopt open source:
- No million-dollar licenses: You're not paying per seat or per user. R is free.
- No vendor lock-in: You own your code and your workflows. You can switch cloud providers or hosting solutions anytime.
- Same ecosystem as global leaders: Roche uses {admiral} and so can you. The packages are open source and validated.
- Transparency: Open source promotes collaboration across companies, academic institutions, and regulatory bodies. Initiatives like Pharmaverse and R Validation Hub share best practices.
In short, company size doesn’t matter. Any company can use the same tools and technologies as the big players.
Benefits for mid-size pharma
Open source gives smaller teams capabilities that used to require enterprise budgets. Any mid-sized pharma company now has:
- Lower barriers to entry: Same validated workflows without six or seven figure software costs.
- Faster deployment: Cloud-native SCEs deploy in weeks.
- Modern talent pool: New hires come trained in R, not legacy systems like SAS.
- Real flexibility: You can use multiple tools and integrate with any environment you need.
- Knowledge sharing: When Roche creates a package to solve a problem, you get insights into their challenges and solutions. You're learning from the best without paying for consulting.
Real-world evidence
Small and mid-size pharma companies are already running trials, building pipelines, and submitting to regulators with R.
Here’s a concrete example from our client who implemented a modern analytics platform and $930,000/year saved in software licensing and 85% drop in AWS compute costs:

The example was extracted from our free eBook - The Anatomy of Modern Statistical Computing Environments in Pharma.
The challenge is knowing how to configure the validated, compliant, and regulatory-ready technology that’s cloud native and designed for pharma workflows. Let’s discuss that challenge next.
How to Make It Happen - Your System for Open Source Clinical Workflows
Moving from "should adopt open source" to "running validated R workflows" requires the right system. In other words, a modern SCE.
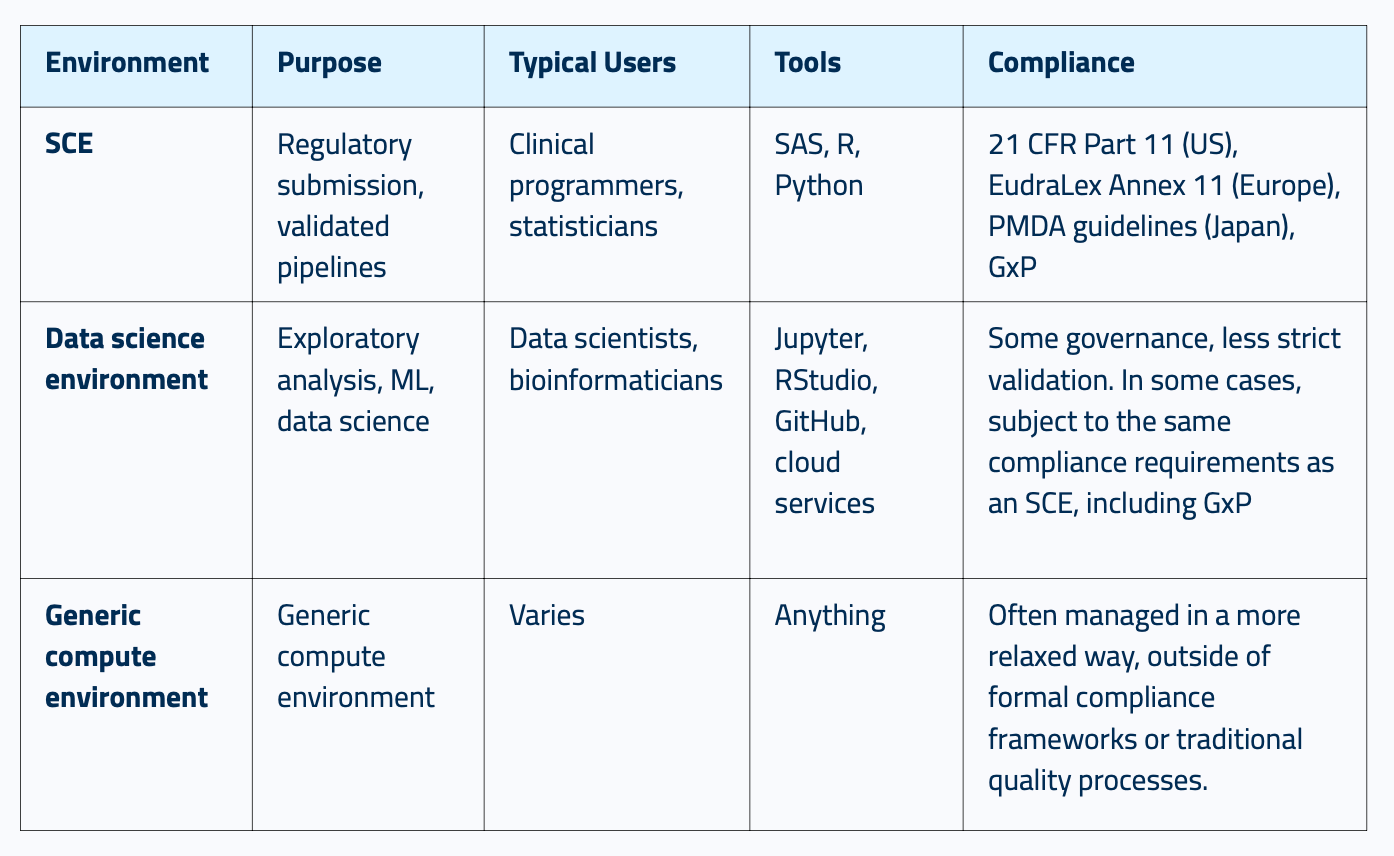
What is an SCE?
In plain English, an SCE (Statistical Computing Environment) allows analytical and statistical workloads to be run and workflows to be developed.

It's a compliant, scalable system built for clinical data analysis that meets regulatory requirements from day one.
If you’re new to the concept of an SCE, here’s exactly how it compares to your traditional data science environment and a generic compute environment:
Managing the transition
Here's what the timeline looks like:
- Assessment phase: 2-4 weeks
- Pilot/PoC: 4-8 weeks
- Full rollout: 8-12 weeks
- Total time to production: 3-6 months instead of 12-18 months
Training and adoption depend on your team's background. Data scientists already know R and can start immediately. SAS programmers will need structured training programs, but with the right resources, they can become productive in R faster than you think.
Next, let’s discuss risk.
We’ll be honest - there is some - like in every transition, but it’s easy to mitigate. You can:
- Run parallel validation during the transition period
- Start with low-risk, high-value pilot projects (non-submission work first)
- Maintain SAS for legacy work while you rewrite workflows in R
- Document everything
As a general rule, don't try to migrate everything at once. Don't skip the pilot phase. Don't under-invest in training. These mistakes will cost you months and kill team morale.
Appsilon's SCE - built for open source in pharma
Appsilon's SCE is designed for pharma companies making the switch to open source.
It’s built on proven open source tools, such as Posit, Kubernetes, and cloud infrastructure. It has a modular architecture, meaning updating individual components won’t break your workflows. And most importantly, it’s secure, auditable, and reproducible by design.
We’ve built it to be:
- Multi-language ready: R, Python, and SAS are pre-configured
- Cloud-native: Scales with your needs and reduces infrastructure costs
- GxP compliant: Validation built in from day one
- AI-integrated: Tools to accelerate app development
In short, you get a system that's ready for regulatory work without building everything from scratch.
You can learn more about it on our official Appsilon SCE page. If you have any questions, our experts will be happy to help.
Conclusion
Open source is proven by global leaders like Roche, Novo Nordisk, and GSK. It's accessible to every pharma company, regardless of size or budget. Appsilon's SCE is the bridge to safe and effective adoption.
Companies that plan to stay on top of their game are moving forward with validated, modern infrastructure that lets them compete on innovation, not licensing costs. Legacy systems are getting left behind.
The good news for mid-sized pharma is that you don't need to be big as Roche to benefit from open source. You just need the right system - built for pharma, compliant with regulatory standards, and scalable as your team grows.
Ready to explore what open source can do for your clinical workflows? Get in touch with our experts to discuss your SCE plans.
FAQ
Is R accepted by the FDA for regulatory submissions?
Yes. Novo Nordisk submitted the first R-based FDA submission, and companies like Roche and GSK now use R for regulatory work. The FDA accepts open source pharma tools as long as your workflows are validated, documented, and reproducible.
How long does it take to set up an SCE?
A modern SCE can be deployed in 3-6 months, including assessment, pilot, and full rollout. This is much faster than traditional 12-18 month implementations. Cloud-native SCEs deploy even faster because they don't require extensive on-premises infrastructure setup.
What's the biggest risk in adopting open source?
The biggest risk is poor implementation. The technology itself is great. If you skip validation, rush training, or try to migrate everything at once, you'll create compliance gaps and frustrated teams. Run parallel validation, start with pilot projects, and invest in proper training to avoid these problems.
Can we run SAS and R in parallel during the transition to open source?
Yes, and you should. Running SAS and R in parallel lets you validate that both systems produce identical results. Novo Nordisk used this approach for their first R-based FDA submission. Keep SAS for legacy work while you gradually rewrite clinical workflows in R or Python.
What's the best way to adopt open source if our team doesn't know R?
Start with structured training programs and low-risk pilot projects. Data scientists can jump in immediately since R is an industry standards. SAS programmers need training, but they can become productive in R faster than expected with the right resources. Just don't skip the training phase - it really makes all the difference.
